Can’t-Miss Takeaways Of Tips About How To Write Empirical And Molecular Formulas

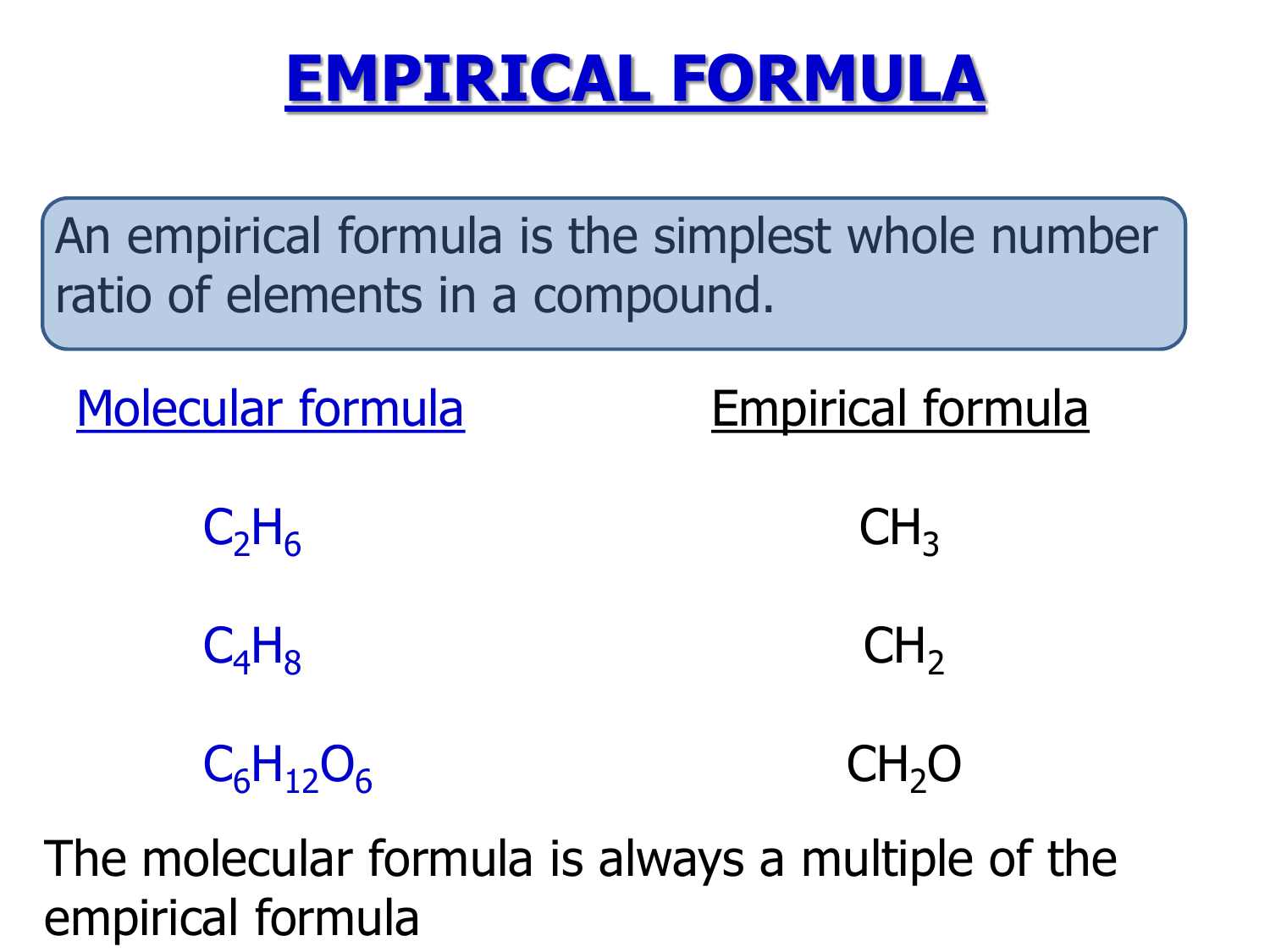
An empirical formula tells us the relative ratios of different atoms in a compound.
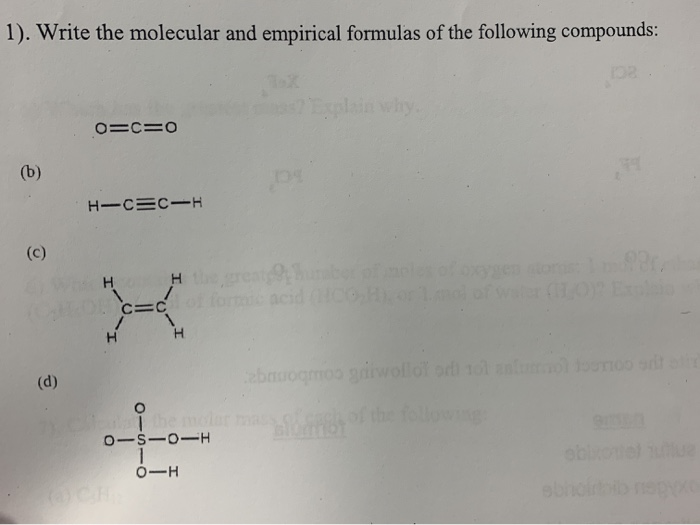
How to write empirical and molecular formulas. Elemental composition of pure substances. Determine the molecular formula of a compound. General chemistry 1 (kattoum) text.
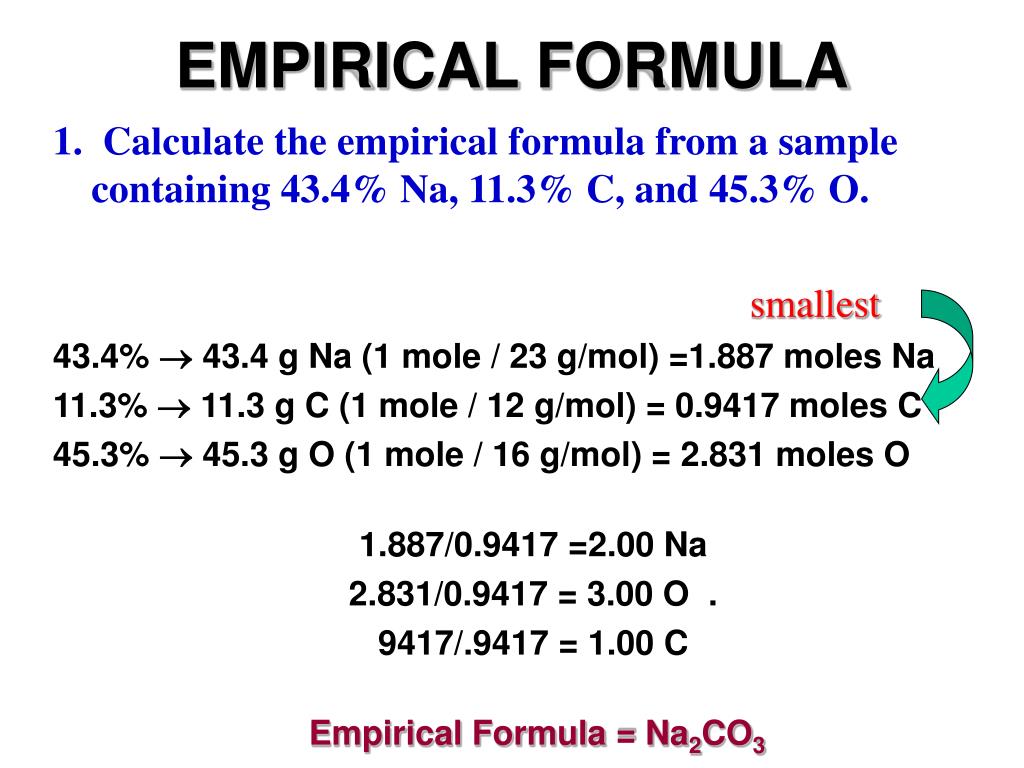
Determine the empirical formula of a compound. \ (\begin {array} {l}= \frac {27.66} {13.81} = 2\end. Molecular formulas tell you how many atoms of each element are in a compound, and empirical formulas tell you the simplest or most reduced ratio of.
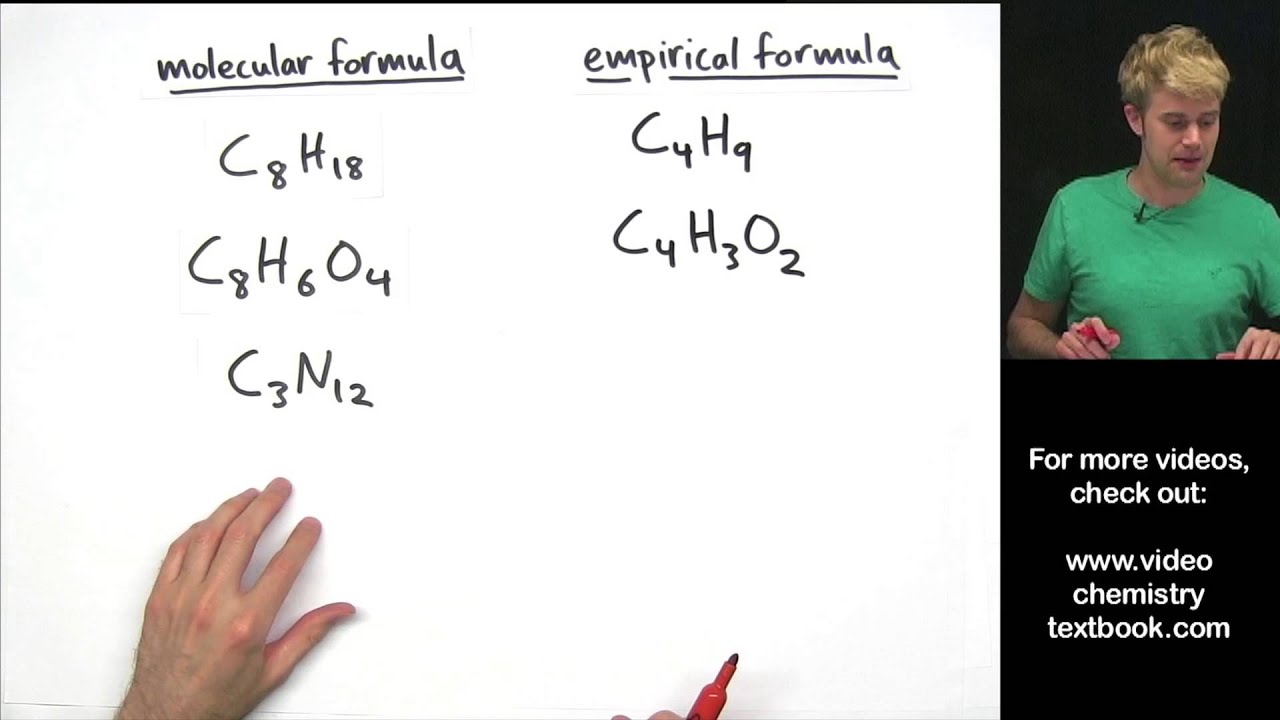
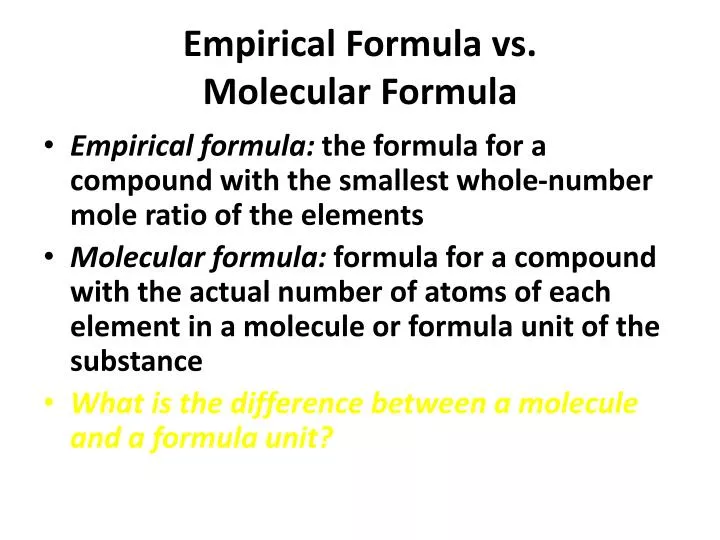
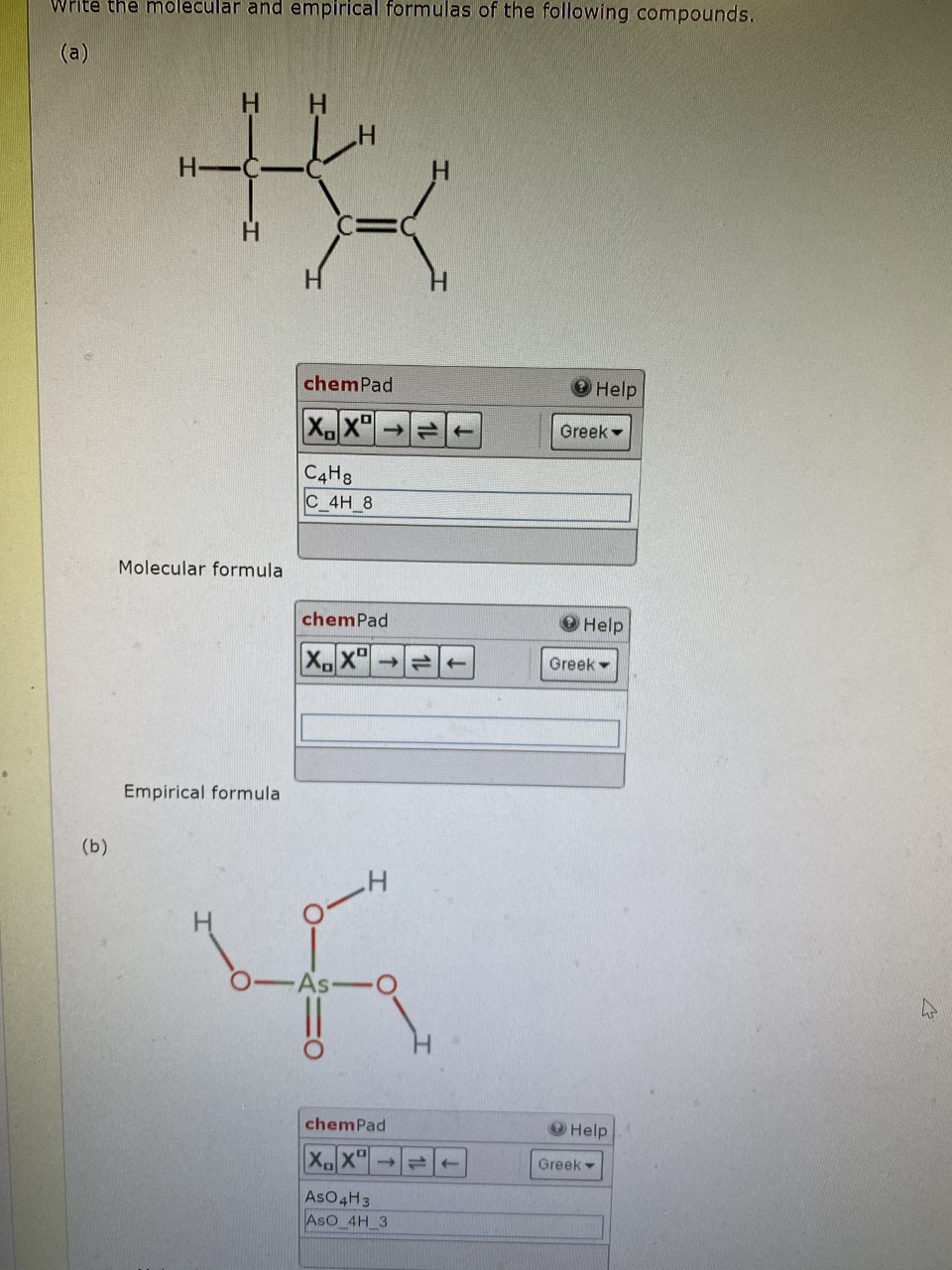
The full formula of a molecule. It states how many of each element in the molecule. Molecular and empirical formulas.
This tutorial covers how to determine the empirical and molecular formulas of a compound from quantitative analyses and includes examples of how to calculate the. In a molecular formula, it states the total number of atoms of each element. The previous section discussed the relationship between the bulk mass of a.
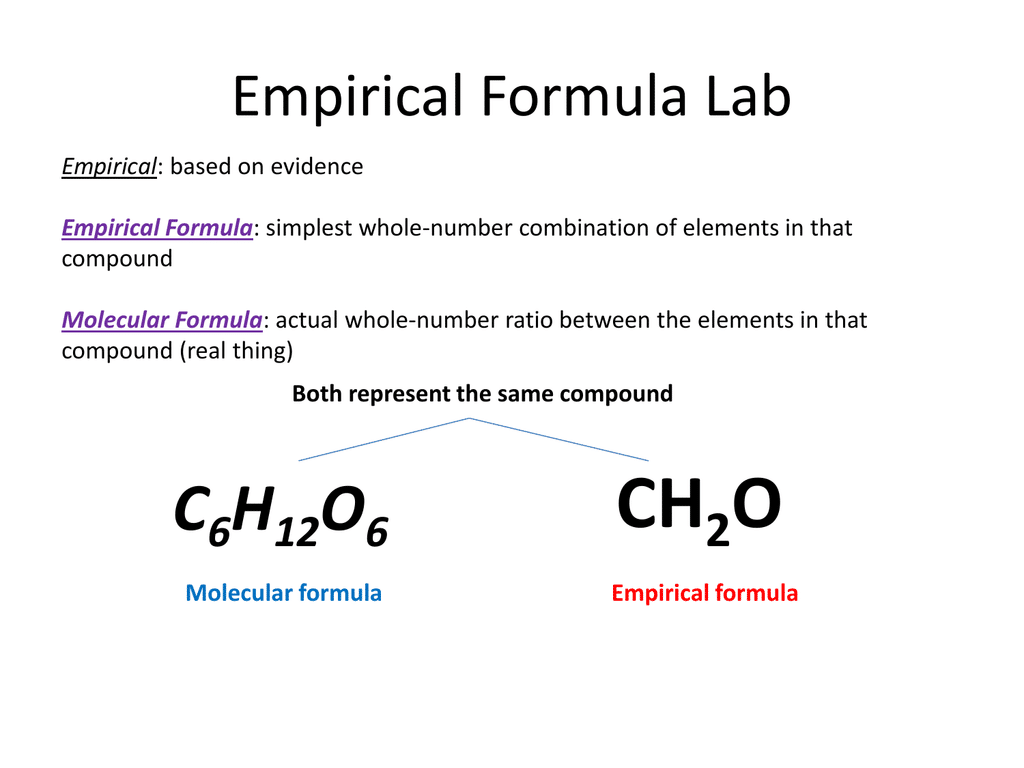
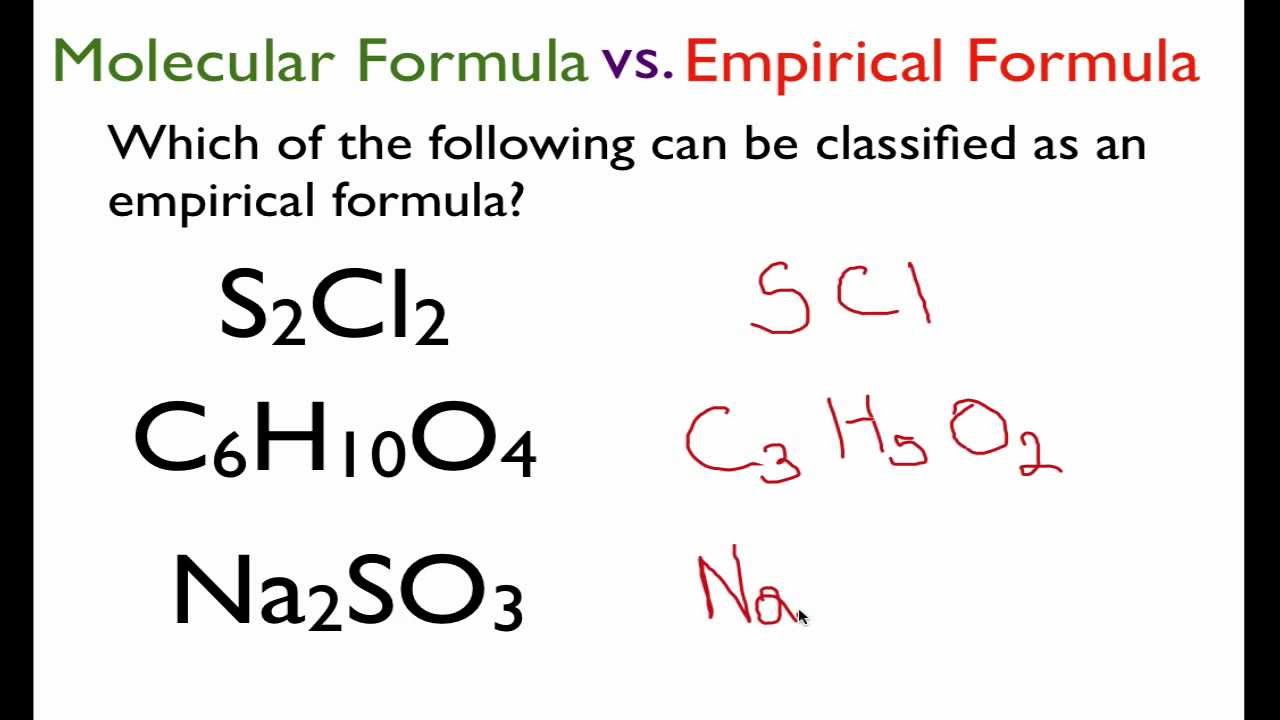
Of a compound is the simplest, whole number ratio of atoms of each element in a compound. Relative empirical mass = (c x 4) + (h x 10) + (s x 1) relative empirical mass = (12 x 4) + (1 x 10) + (32 x 1). Thus, h 2 o is composed of two atoms of hydrogen.
The empirical formula of a compound is the simplest formula of a compound that shows the atoms in their simplest ratio. This chemistry video tutorial explains how to find the empirical formula given the mass in grams or from the percent composition of each element in a compound. By using the expression, molecular formula = n × empirical formula.
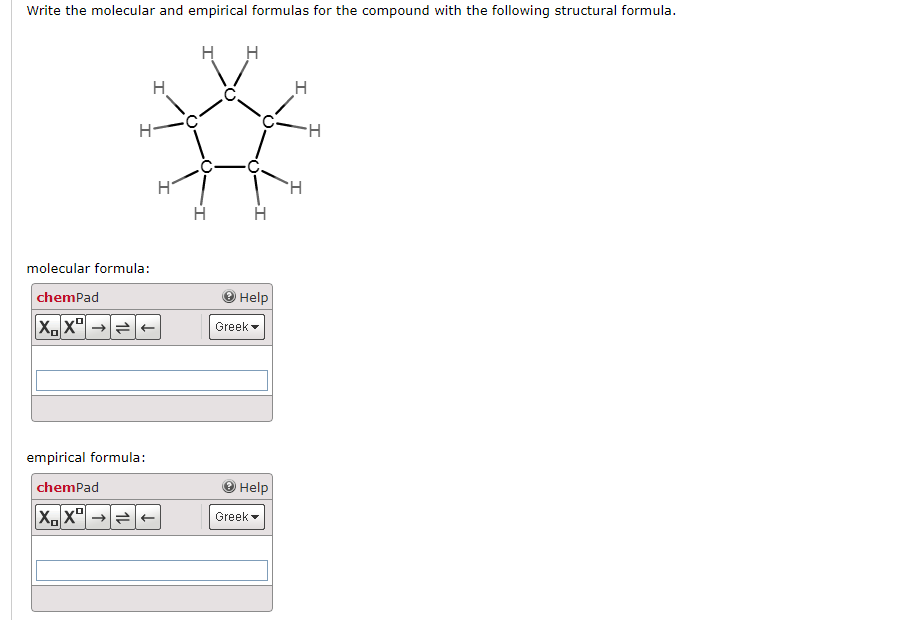
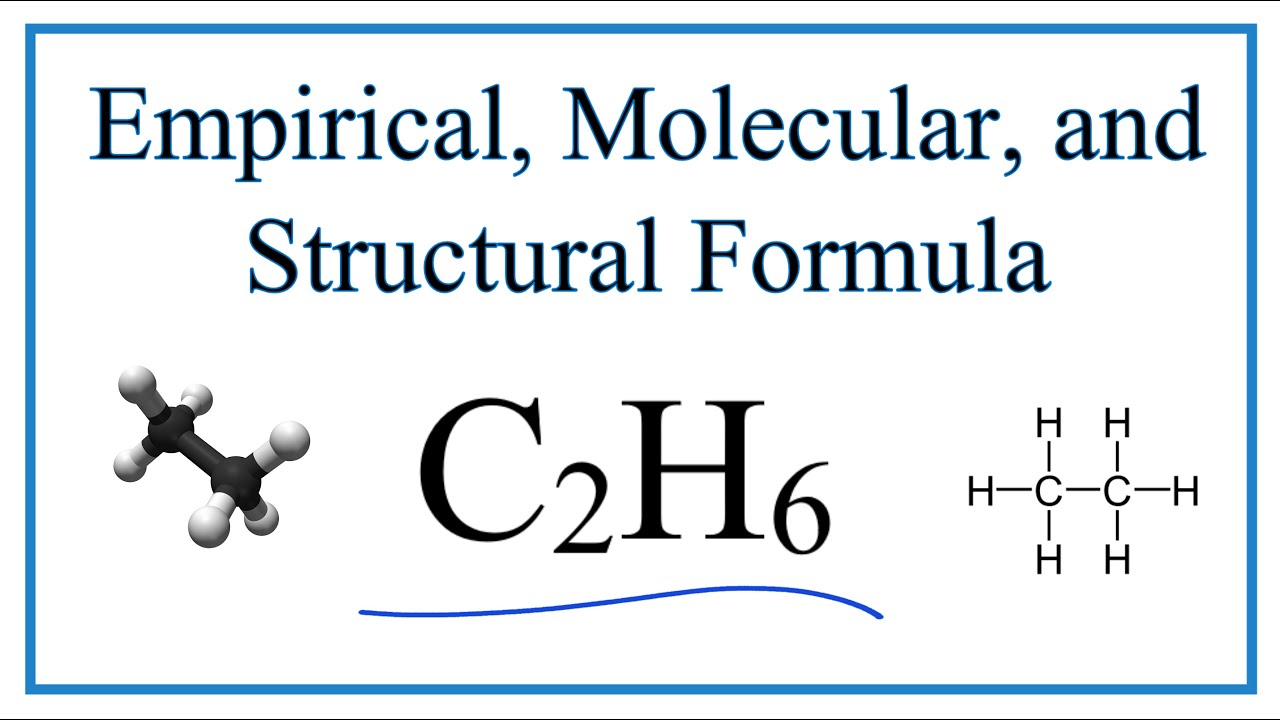
Empirical, molecular, and structural formulas. It is the smallest whole number ratio of atoms, but does not necessarily represent the arrangement of atoms in. Calculate relative mass of the empirical formula.
1.6m views 9 years ago. The empirical formula for glucose is ch 2 o. The subscripts are whole numbers and.
Write the empirical formula. Shows the actual number of atoms of. The empirical formula is the simplest formula of a compound.
The empirical formula of the compound is \(\ce{fe_2o_3}\). The ratios hold true on the molar level as well. Oregon institute of technology.
.PNG)
.PNG)